An III 2013
Third stage report
II.2., II.3., II.4., II.5. si II.6. Synthesis of cycloadducts derived from imidazolium salts (obtained in stage II.1.) by [3+2] dipolar cycloaddition with activated alkynes and alkenes.
In this stage was investigated the reaction of the benzimidazolium salts 5d with dimethylacetilenedicarboxilate (DMAD). We have done first the reaction in chloroform, and the reaction product is the totally aromatized and in the same time with the ester group from the first position hidrolized 6.
In order to elucidate the influence of the solvent over the hydrolysis that occurs, we repeated the reaction in benzene and in methanol. In benzene we obtained only the hydrolyzed product 6, while in methanol we have obtained both hydrolyzed 6 and un-hydrolyzed cycloadduct 7. Un-hydrolyzed product is the minor product.

Recently, due to the fact that we had access to an X-ray diffractometer, we were able to determine the exact structure of compound 6 which turned out to be:
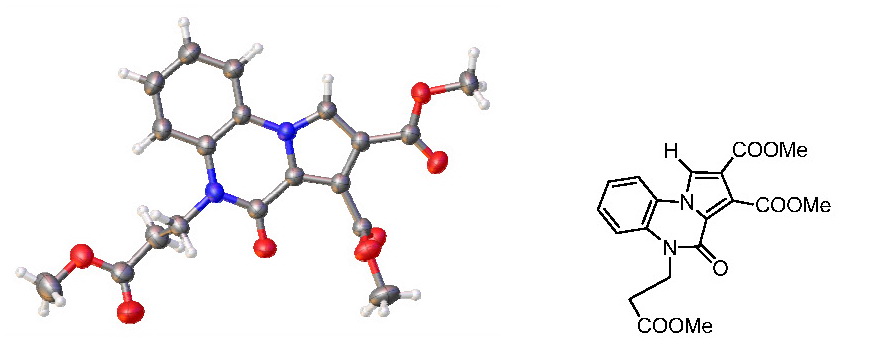
A re-analysis of the NMR spectra has determined that the signals of the protons and carbons of the spectra were consistent with the proposed structure.
The structure of the compound 6 being established precisely we raised the question of the reaction mechanism by which this compound is obtained. We assumed that the cycloaddition reaction conclude to an unstable imidazo-dihydropyrrolo derivative, with a pronounced tendency for aromatization of the pyrrole ring. In the aromatization process the imidazole ring is opened, the pyrrole ring is aromatizated by tautomerism, then is followed by a pyrrole ring rotation of 180 degrees around the N3-C4 bond (from the initial imidazole ring). Finally there is a new 6-membered ring closure by elimination of a methanol molecule.
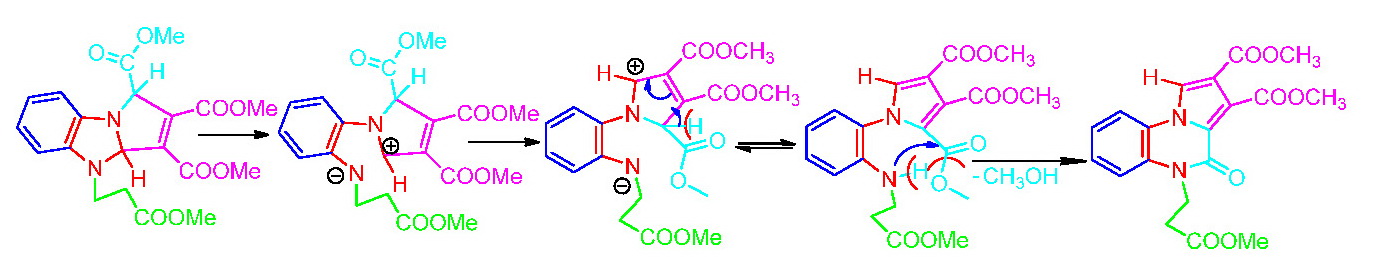
In order to confirm the proposed mechanism was also investigated the reaction of salts 5a, 5b, 5c and 5e with dimethylacetylenedicarboxylate (DMAD).
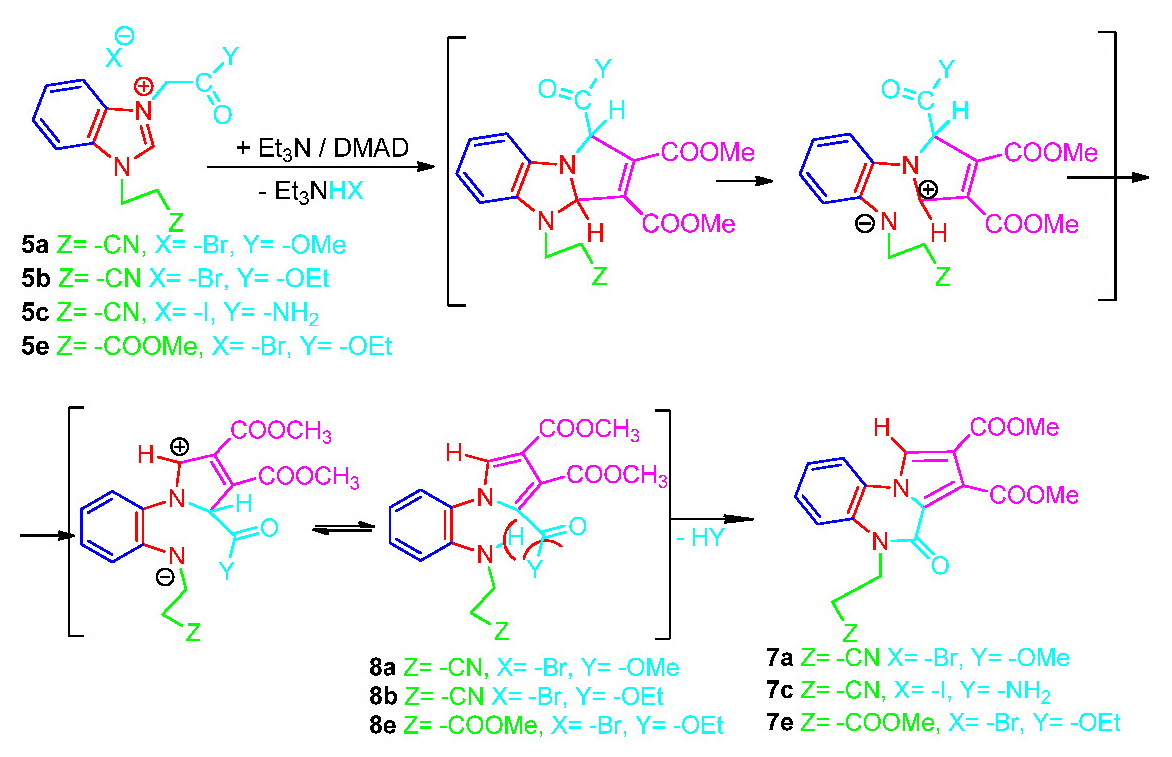
In the case of the salts 5a and 5e were identified and isolated both 7a and 7e cyclized products and 8a and 8e products with aromatic pyrrole ring but with imidazole ring open. In the case of the salt 5c was isolated only cyclized product 7c while in the case of the 5b salt we have isolated only the intermediate 8b. Moreover for this intermediate we managed to register the X-ray spectrum, and the structure we found is the same with the proposed structure and thus confirms the proposed mechanism:

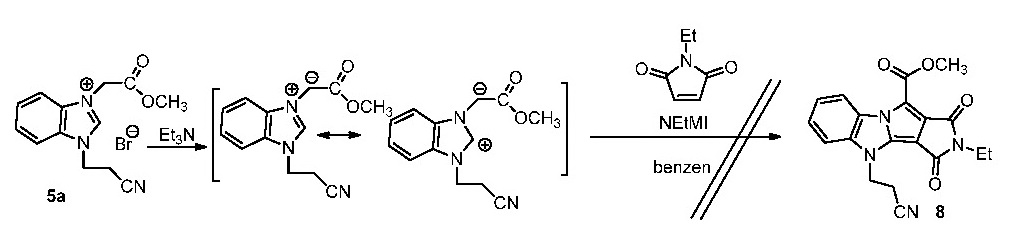
The way the aromatization of the cycloaddition product take place (by pyrrole ring opening) explains the failure of isolation of the cycloaddition products in the case of imidazolium salts and even benzimidazolium salt in the case of the reaction with the activated alkene. Previously has been investigated reaction of the salt 5a with N-ethylmaleinimide (NEtMI). Unfortunately, the attempts to separate and identify the reaction products have failed.
III.1. Synthesis of new imidazolium hexafluorophosfaes from corresponding halides (obtained in stage II.1.) by anion metathesis.
We managed the reaction of the salts 5a and 5c with aqueous solution of KPF6.

In the case of the salt 5c the reaction was successful, In the case of the salt 5a we have encountered difficulties in removing the potassium bromide formed in the reaction since hexafluorophosphate 9a is also soluble in water. In order to eliminate this drawback we decided to use another methodology anion metathesis, a methodology that does not require the use of aqueous solution and in which the halide elimination is easier. So we decided to apply a method found in literature that involves treating imidazolium halides with dimethyl sulfate, and we treated the salt 4f with dimethyl sulfate. The NMR spectrum of the isolated product was consistent with the proposed structure for methylsulfate 10f.
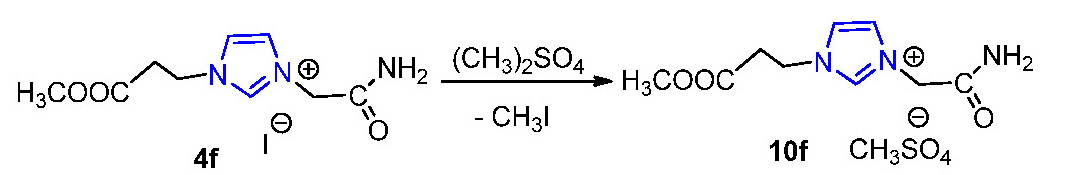
In present we extend this reaction to the other imidazolium halides.
III.2. Synthesis of the aliphatic spacers.
Aliphatic spacers were obtained by the reaction of the chloroacyl chlorides (chloroacetyl, chloropropionyl and chlorobutiryl) with diamino derivatives (1,2-diaminoethane, 1,3-diaminopropane, 1,4-diaminobutane and 1,6-diaminohexane).
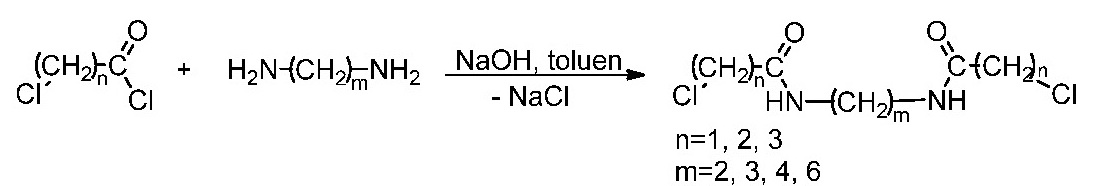
The compounds were synthesized and spectral characterized.
III.3. Synthesis of the aromatic spacers.
Aromatic spacers were obtained by the reaction of the chloroacyl chlorides (chloroacetyl, chloropropionyl and chlorobutiryl) with aromatic diamines (o-, m- and p-phenylene diamine).
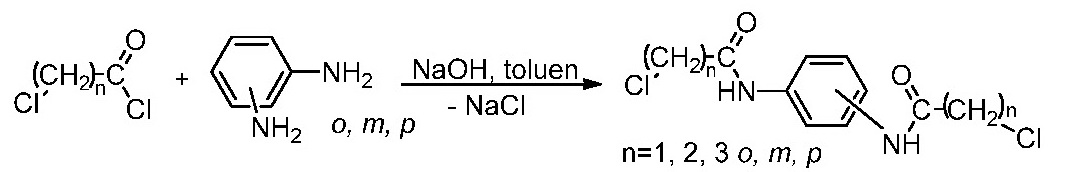
The compounds were synthesized and spectral characterized.
III.4. Synthesis of the bis-imidazo derivatives with aliphatic spacers.
Bis-imidazo derivatives with aliphatic spacers were obtained by the reaction of the aliphatic spacers (obtained in stage III.2) with sodium imidazolate.

The compounds were synthesized and spectral characterized.
III.5. Synthesis of the bis-imidazo derivatives with aromatic spacers.
Bis-imidazo derivatives with aromatic spacers were obtained by the reaction of the aromatic spacers (obtained in stage III.3) with sodium imidazolate.

The structure of the new compounds was or will be chemical (elemental analysis) and spectral determined (IR, UV-VIS, NMR, MS).
The structure of the compounds was proved for all imidazole derivatives by elemental analysis and spectral analyses. Were recorded and solved IR, UV-VIS, RAMAN, MS, RMN and DRX spectra. All the elemental and spectral data are in accordance with the proposed structure.
Project’s web page: http://teclu.chem.uaic.ro/moldoveanu/contract-te/, was continuous updated with the scientific reports.
A part of the obtained results were organized to be published in journals and presented on a series of national and international conferences as following:
One paper accepted for publication:
- D. Astefanei, N. Buzgar, M. Risca, C. Moldoveanu, I. Mangalagiu: „Synthesis, SERS, Raman and FT-IR investigation in conjunction with DFT theoretical simulations on N-(2-cyanoethyl)-imidazole. Part I”; Revista de Chimie, 64(x), xxx-xxx, 2013. (ISSN: 0034-7752) accepted.
4 papers presented on national and international conferences:
- C. Moldoveanu, Ghe. Zbancioc, D. Mantu, P. M. Stiuleac, I. O. Silea, I. Mangalagiu Sinteza de noi lichide ionice cu schelet imidazolic Zilele Universitatii “Al. I. Cuza-Iasi“, 31 Octombrie – 1 Noiembrie 2013, (Poster ).
- Ghe. Zbancioc, C. Moldoveanu, D. Maftei, V. Antoci, I. Mangalagiu: Sinteza şi elucidarea structurii unor noi derivati de pirrolo-imidazol fluorescenti Zilele Universitatii “Al. I. Cuza-Iasi“, 31 Octombrie – 1 Noiembrie 2013, (Poster ).
- Mantu, D.; Moldoveanu, C.; Zbancioc, G.; Antoci, V.; Stoian, I.; Mangalagiu, I.: „Synthesis of new ionic liquids imidazole based” XIV European Symposium on Organic Reactivity ESOR 2013, 1-6 September 2013, Prague, Czech Republic. (poster 69).
- Zbancioc, G.; Maftei D.; Moldoveanu, C.; Zbancioc, A.M.; Tataringa, G.; Mangalagiu, I.: „Synthesis and XRD Structure Elucidation of new Fluorescent Pyrrolo-imidazole” XIV European Symposium on Organic Reactivity ESOR 2013, 1-6 September 2013, Prague, Czech Republic. (poster 137)
In present we work on data organization in view of the publication of two papers in ISI cited journals.